Step-by-step guide
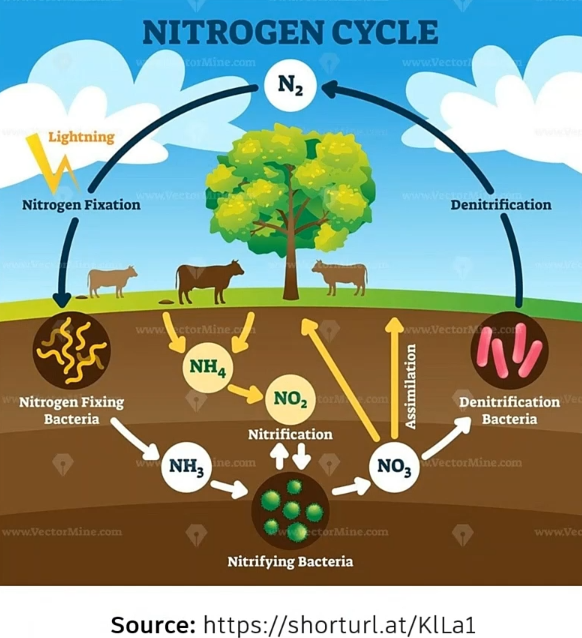
Ammoniacal nitrogen and the processes of nitrification and denitrification are crucial components of the nitrogen cycle and are vital to the health of aquatic and terrestrial ecosystems.
Ammoniacal nitrogen can originate from industrial, agricultural, or natural sources.
Understanding its transformation in the environment is essential for managing water quality and maintaining ecosystem health.
Ammoniacal nitrogen:
- Consists of nitrogen in the form of ammonia (NH3) or ammonium ions (NH4).
- Enters ecosystems through industrial or agricultural runoff, decomposition of organic matter, and direct excretion.
In aquatic systems, bacteria convert ammoniacal nitrogen first to nitrite (NO2) and then to nitrate (NO3). This oxidation process consumes dissolved oxygen and is a crucial part of the biochemical oxygen demand cycle.
Nitrification:
- Converts ammonia into nitrite and then nitrate under aerobic conditions, which is vital to the nitrogen cycle.
- Transforms ammonia, which can be toxic in high concentrations, into nitrate, a form that plants can easily absorb.
- Consumes dissolved oxygen, highlighting the need for adequate oxygen levels to maintain water quality.
The bacteria responsible for this process are Nitrosomonas, which oxidize ammonia to nitrite, and Nitrobacter, which further oxidize nitrite to nitrate.

Denitrification:
- Occurs under low oxygen or anoxic conditions, typically when dissolved oxygen is less than five percent of its saturation value.
- Relies on bacteria, which use nitrates and nitrites as oxygen sources to meet their biochemical oxygen demand.
- Results in the release of nitrogen gas into the atmosphere, completing the nitrogen cycle.
Denitrification is essential for reducing excess nitrogen in water bodies.
Next, to simulate ammoniacal nitrogen in a 1D river model, create a pollutograph. In this example, a pollutograph has already been created.
- In the Explorer window, right-click the pollutograph and select Open to review the definitions.
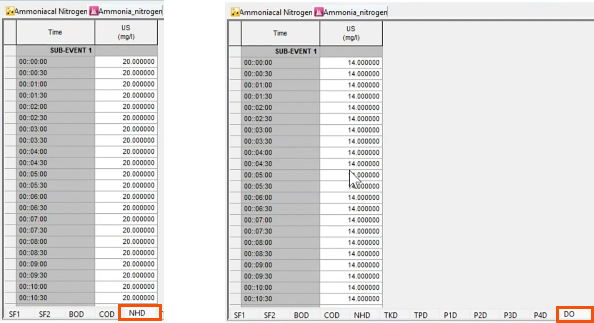
- In the pollutograph, select the NHD tab to assign a concentration. Here, 20 milligrams per liter of ammonia is assigned to the US node.
- On the DO tab, assign a concentration of 14 milligrams per liter of dissolved oxygen to the US node.
- In the Run dialog, add the pollutograph.
- Name the run accordingly, such as “Ammoniacal Nitrogen”.
- Click QM parameters.
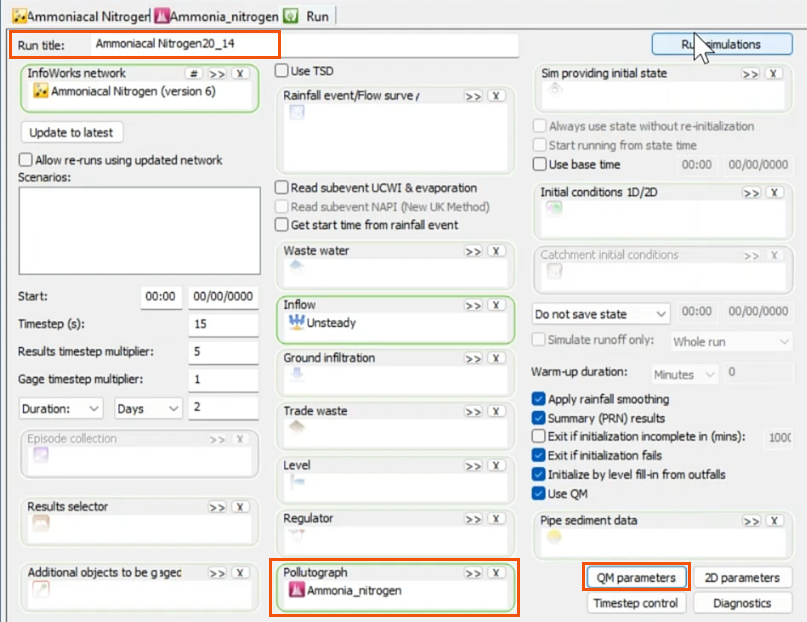
- In the QM Parameters dialog, select BOD, COD, TKN, NH4, DO, NO2, NO3, and TW as determinants.
- Click OK.
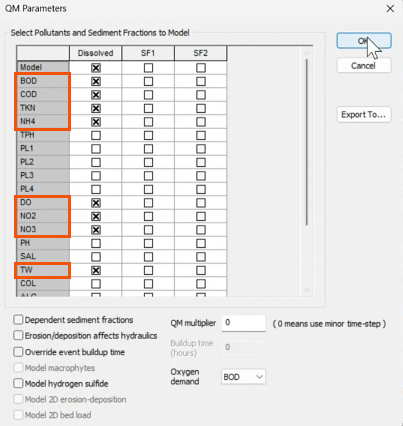
- In the Run dialog, click Run simulations.
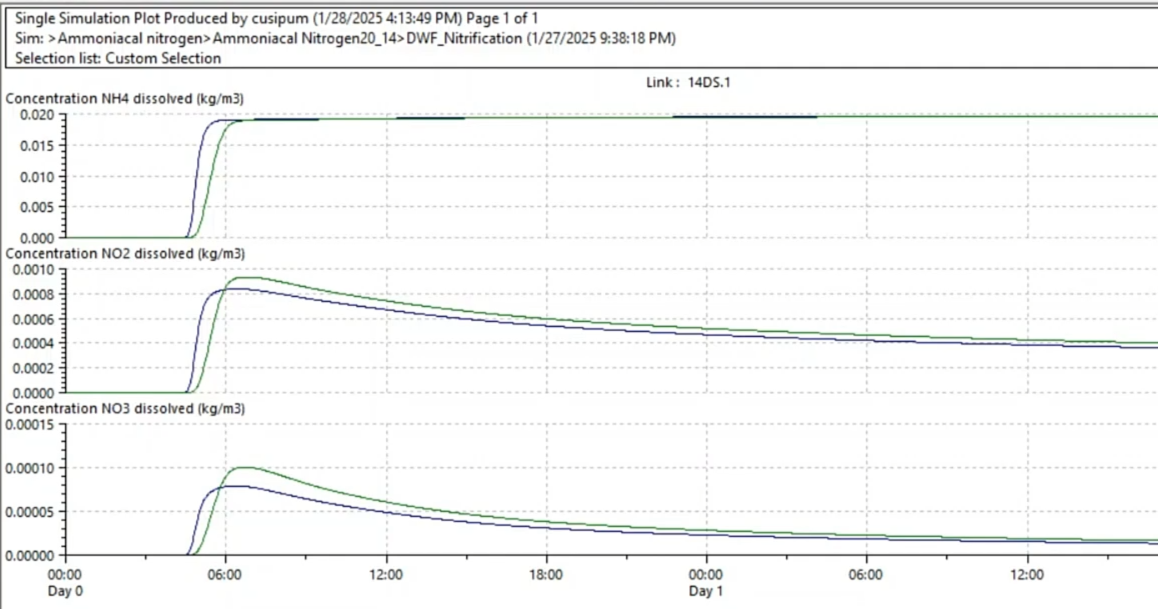
Now, analyze the results to observe how ammonia (NH4) is oxidized, leading to the formation of nitrite (NO2) and nitrate (NO3).
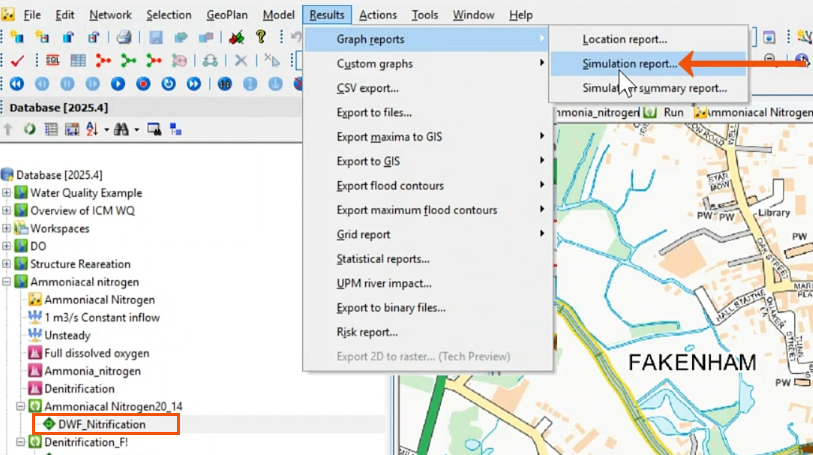
- In this case, open the results of a previously run simulation, DWF_Nitrification.
- Select Results > Graph reports > Simulation report.
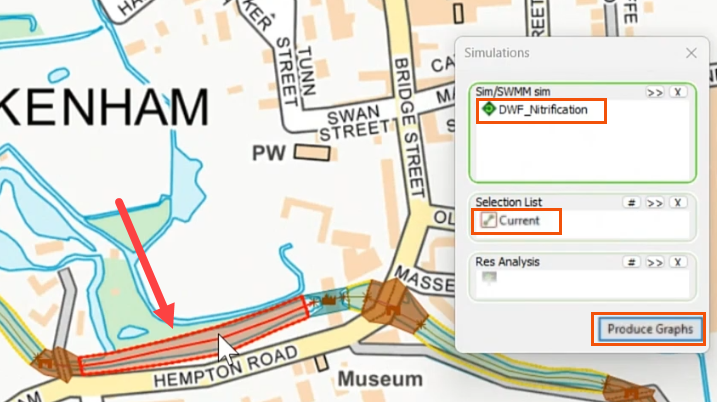
- Drag DWF_Nitrification into the Simulations dialog.
- In the GeoPlan, select the river reach.
- In the Simulations dialog, apply the Current selection to the Selection List.
- Click Produce Graphs.
- Review the results.
Review the results:
Next, to simulate a denitrification process that occurs under low oxygen conditions, create another pollutograph called “Denitrification”. In this example, it is already created.
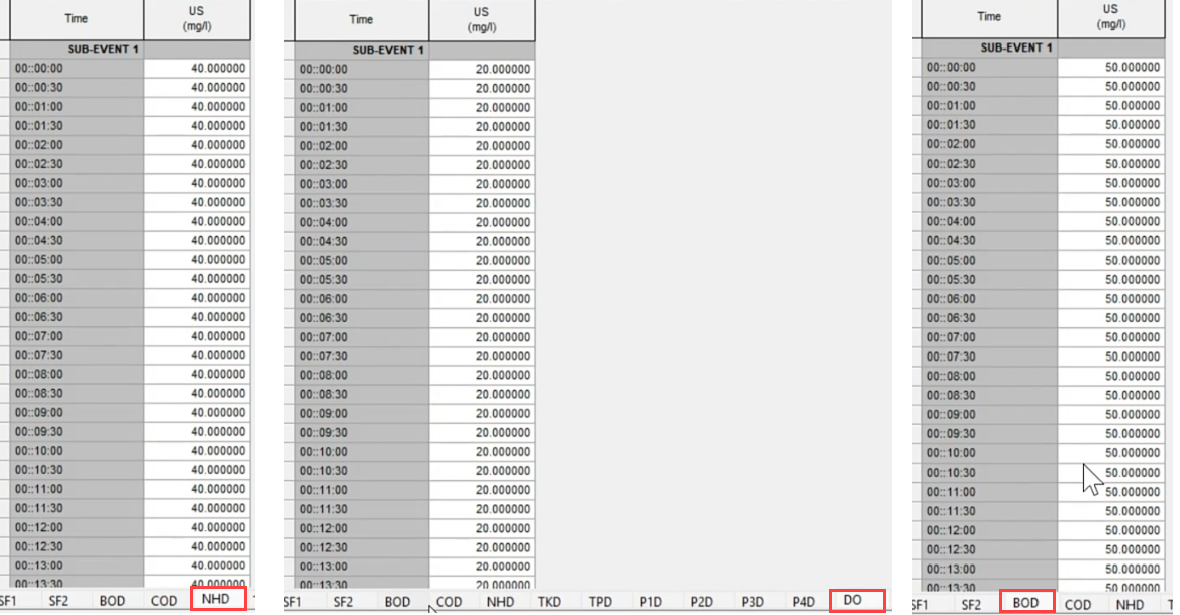
- Assign the following concentrations to the upstream node:
- Set NHD at 40 milligrams per liter.
- Set DO at 20 milligrams per liter.
- Set BOD at 50 milligrams per liter.
- Again, open the Run dialog to add the pollutograph, name the run, and select the QM parameters.
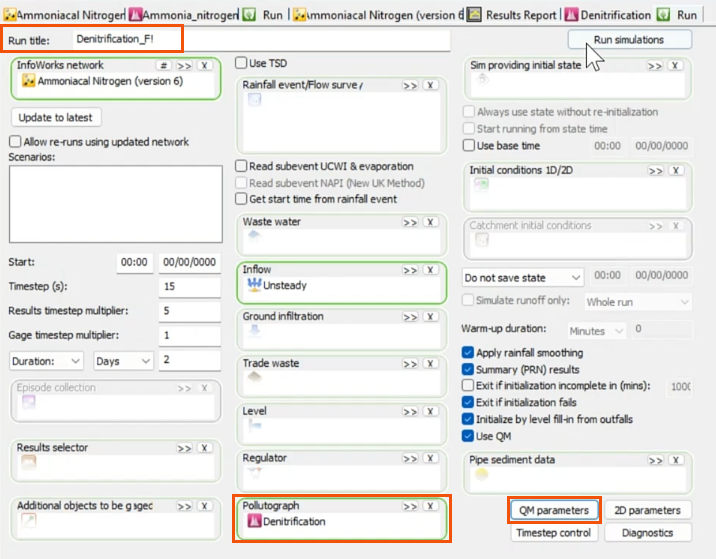
- In the QM Parameters dialog, include the same determinants as the previous run.
- Run the simulation.
Now, analyze the results.
- Open the simulation results.
- In the Parameter Selection dialog, select Concentration NO2 dissolved, Concentration NO3 dissolved, and Concentration NH4 dissolved.
- Deselect Include Rainfall.
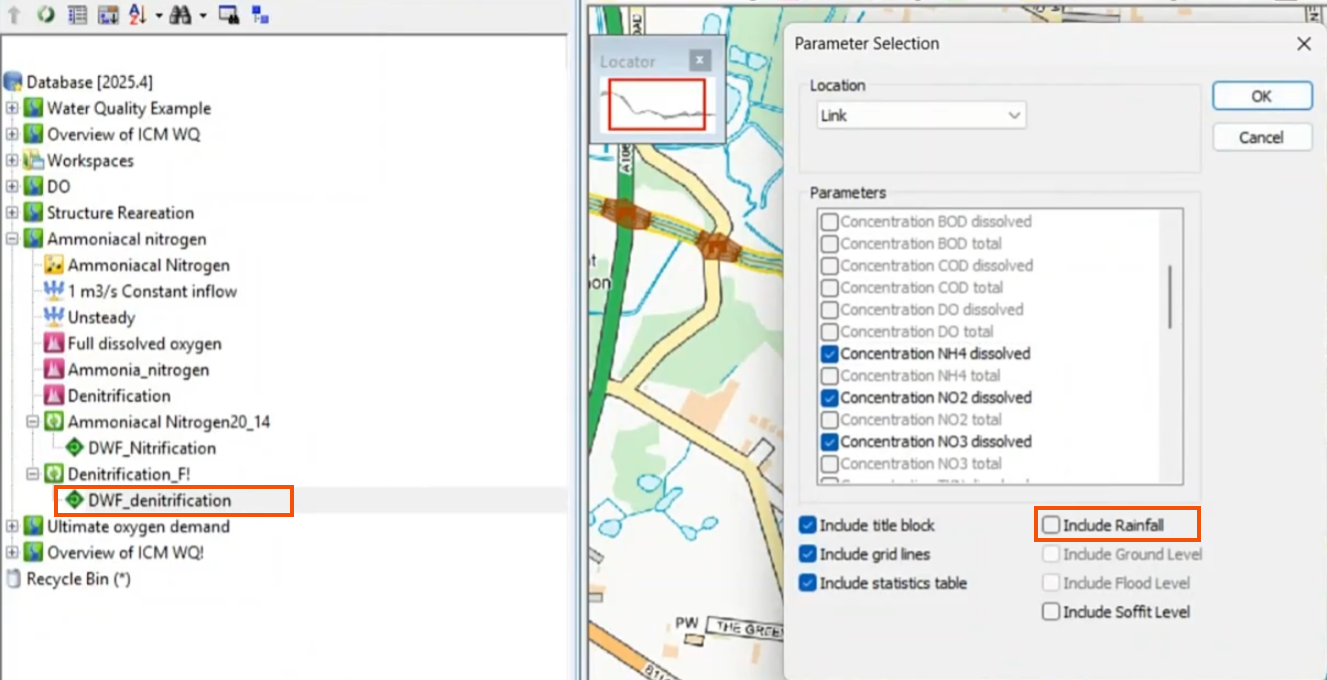
- Click OK.
- In the Simulations dialog, add the denitrification simulation and select Current for the Selection List.
- Click Produce Graphs.
Review the results:

During the first five hours, when DO levels are high, ammonia undergoes oxidation to nitrite and nitrate. However, after five hours, DO concentrations decline, causing nitrification to stop.
At this stage, denitrification takes over, and NO2 and NO3 are used to meet BOD requirements, leading to the release of nitrogen gas (N2) into the atmosphere.
This transition illustrates how oxygen availability influences nitrogen transformations in water bodies. It also highlights the importance of balancing nitrification and denitrification in water quality management.
















